Vizuell: Young Edison Gold Award 2022
I won the gold award with Vizuell at the 2022 Young Edison Awards.
The Problem:
Hand Hygiene compliance in ICUs and non-ICUs[3] less than 50%
Users often do not achieve full coverage of sanitizing agents - provides a false sense of security
COVID can spread through face-touching behavior, which is often frequent and unconscious [9]
However: Proper hand hygiene breaks chain of infection
[3] McGuckin, Maryanne, et al. [9] Kwok et al.
The Solution: Vizuell
Vizuell is a verifiable decolorizing sanitizer.
Two-component system:
Part 1: Iodine-based sanitizer, blue coloration allowing for users to visually verify hand coverage
Part 2: Alcohol-based sanitizer that clears blue color with ascorbic acid
Dual sanitizing components: quick, safe, cheap, effective.
Decolorization Method 1: Fenton’s Reagent
My first attempt at decolorization examined Fenton’s reagent, a reaction commonly utilized in wastewater treatment to remove dyes and pollutants from the textile industry.
The reaction consists of a solution of hydrogen peroxide and ferrous gluconate. Hydrogen peroxide is catalyzed by the ferrous ion to decompose into hydroxyl free radicals. These free radicals are oxidation agents, and are very effective at degrading dyes.
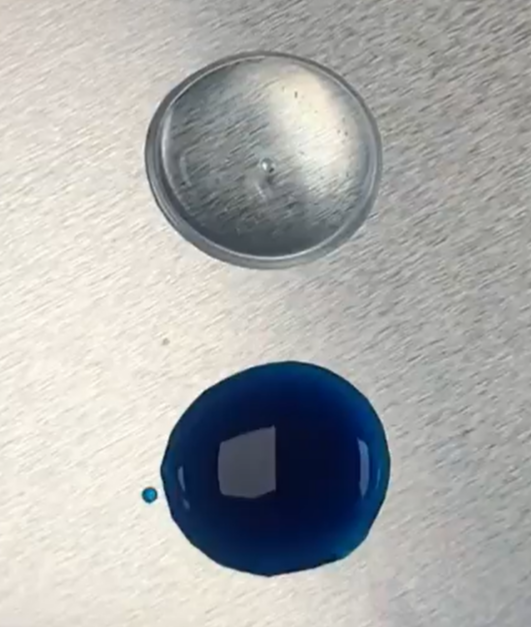
Results:
As seen to the right, the methylene blue solution is scavenged and disappears. However, the decolorized solution still has a greenish/yellow hue characteristic of ferrous gluconate.
Both the methylene blue solution and the hydrogen peroxide solution had a 70% alcohol content, ensuring proper sanitation. However, the lingering color made this method suboptimal.
Decolorization Method 2: Redox Reaction
The second decolorization mechanism utilizes an iodine/ascorbic acid redox reaction.
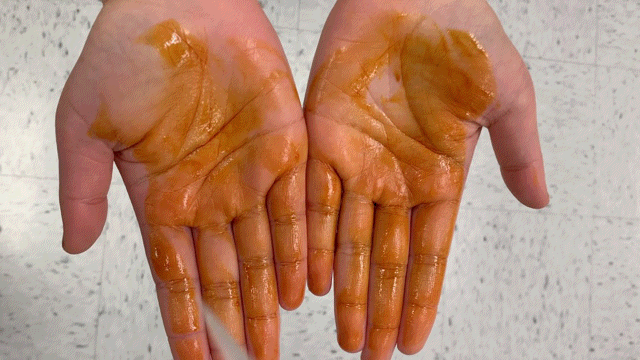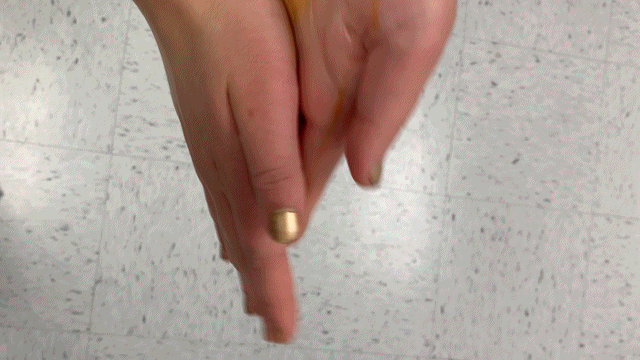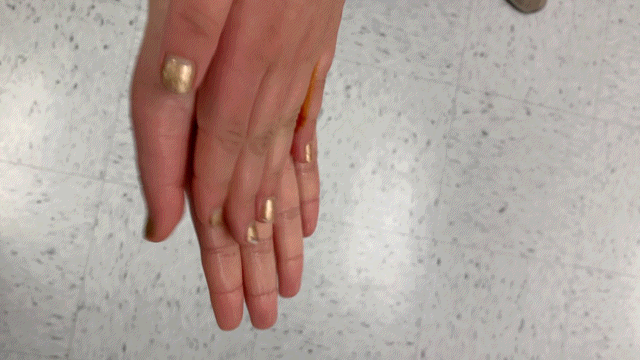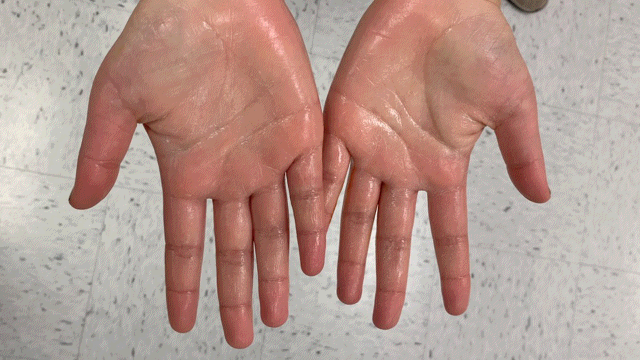
One component contains povidone-iodine, a common wound disinfectant, with strong color. The staining from the Povidone-iodine allows users to ensure total hand coverage, while also providing antiseptic properties.
The second component is an alcohol solution containing Vitamin C, which oxidizes Iodine to Iodide. Ascorbic acid is reduced to form dehydroascorbic acid.
Adding a solution of soluble starch to the iodine mixture creates charge transfer complexes with an intense blue color.
Results:
The blue form of iodine still decolorizes in the presence of ascorbic acid.